
The PI3K/Akt/mTOR pathway is one of the major cellular pathways that plays a key role in basic intracellular functions including cell growth, proliferation, motility, differentiation, and survival. Disruption and abnormal activation of the PI3K/Akt/mTOR pathway are frequently observed in various human malignancies making this pathway an attractive therapeutic target in the treatment of cancer. Inhibition of this pathway has been investigated in a wide range of preclinical and clinical trials, and it becomes more evident that blockade of PI3K/Akt/mTOR leads to regression of human tumors.
Some inhibitors of PI3K/Akt/mTOR pathway are approved by the Food and Drug Administration as effective therapies in cancer treatment.
Four isoforms of Class I PI3K, PI3K α, β, γ, and δ, as well as mTOR complexes, are considered primary targets for the development of anti-cancer small molecule inhibitors. Based on the selected target, several classes of small molecule inhibitors exist, including pan-PI3K inhibitors (inhibit all isoforms), isoform-specific inhibitors, and dual PI3K/mTOR inhibitors. Research and development experts at Torqur aim to create a portfolio of disease-specific mTOR and/or PI3K inhibitors with optimized pharmacokinetic and safety properties. By employing the most promising molecules designed to specifically inhibit desired pathways of cancer cells and minimize possible drug toxicity, Torqur intends to turn cancer into a manageable disease.
Torqur intends to turn cancer into a manageable disease.
Bimiralisib (PQR309)
Our key product is bimiralisib (PQR309), a dual pan-PI3K and mTOR inhibitor. Such simultaneous inhibition of the ATP site of all class-I PI3K isoforms and mTORC1 and mTORC2 is Torqur’s preferred strategy to reduce tumor growth and slows down cancer cell metastasis. Furthermore, blocking the PI3K/mTOR cascade on both levels minimizes the possibility of cancer cells developing drug resistance. Bimiralisib is a small molecular weight chemical compound that penetrates the blood-brain barrier showing potent anti-proliferative in vitro activity against a series of tumor cell lines and in vivo antitumor efficacy. Bimiralisib has been investigated in multiple oncology phase 1/2 clinical trials in various indications where different dosing schedules have been explored. Bimiralisib demonstrated early clinical signs of efficacy and good tolerability with the intermittent dosing schedules enabling the opportunity for further development as a monotherapy and/or in combination with other therapies. Bimiralisib is a small achiral molecule with favorable pharmacokinetic parameters in different preclinical models. Oral administration of Bimiralisib in clinical development showed encouraging activity and acceptable safety profile in advanced solid tumours and R/R lymphoma. Bimiralisib is a suitable candidate for the treatment of other indications. As a brain penetrant, the drug could allow the treatment of brain tumors, brain metastasis, and different neurological disorders. Its topical application could be applied against certain skin cancers and dermatological conditions.
1/4
Allosteric mTOR inhibition
IGF
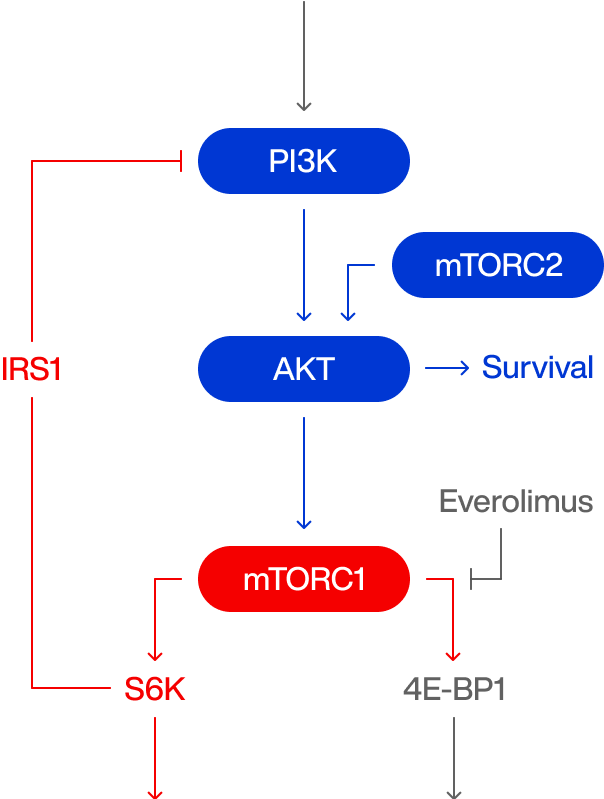
G1 / S arrest
Weak autophagy
Unintended feedback
Immunosuppression
ATP competitive mTOR inhibition
IGF
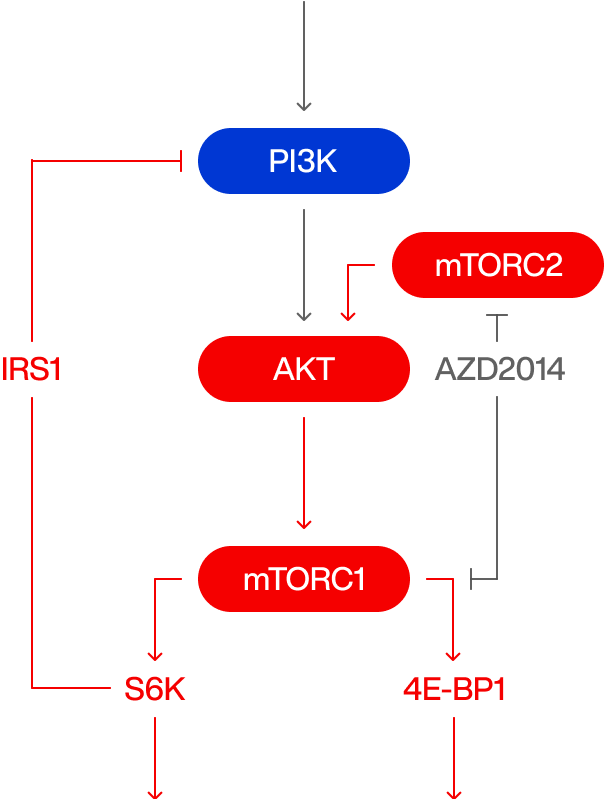
G1 / S arrest
Robust autophagy
No feedback
No Immunosuppression
ATP competitive PI3K inhibition
IGF
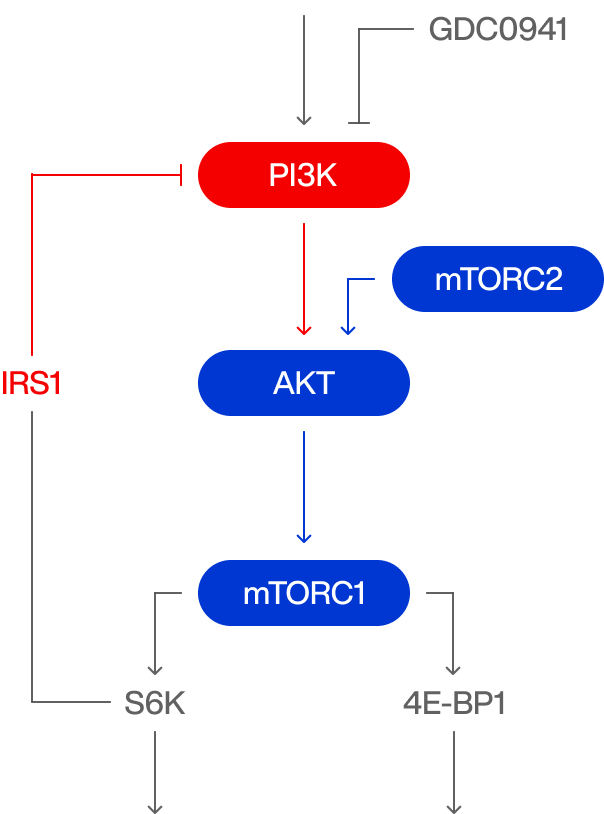
G1 / S arrest
Hyperglycemia
ATP competitive PI3K/mTOR inhibition
IGF
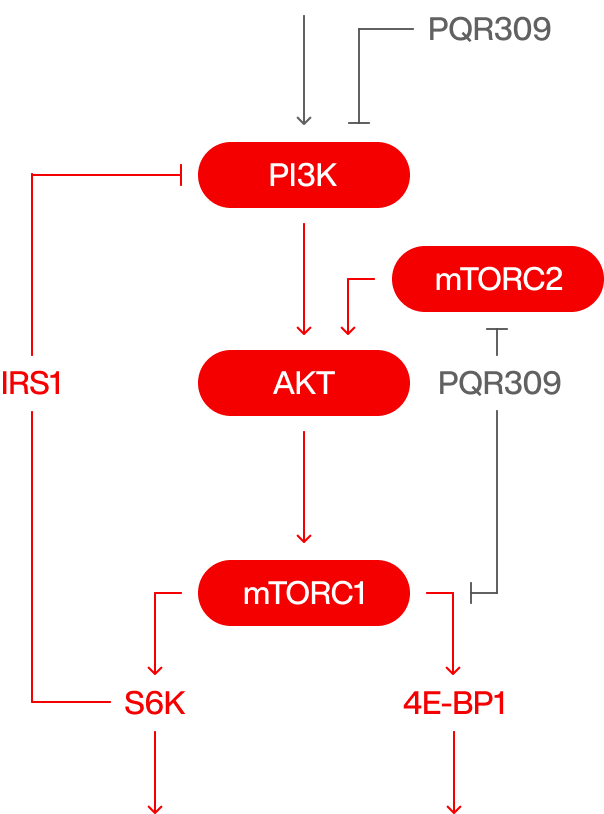
G1 / S arrest
Autophagy
Hyperglycemia
1/4
PIPELINE
Torqur’s patent portfolio protects and covers a large area of lipid kinase (PI3K) and mTOR inhibitor products and applications. Torqur’s intellectual property strategy maximizes the protection of its technologies, know-how, variety of indications, and research areas of current and future interest. Torqur’s differentiation against its peers is the outstanding industry expertise, excellent contacts with universities, the level of innovation with its unique, proprietary fragment and scaffold libraries, and its best-in-class products with the novel PI3K/mTOR inhibitors that fully address the given challenges, meeting therapeutic, tolerance and galenic needs for both oral and topical applications.